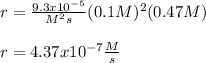The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M and [O2] = 3.7 M, the observed rate of the reaction is 0.00022022 M/s. (a) What is the value of the rate constant? (d) What is the rate of reaction when [NO] = 0.1 M and [O2] = 0.47 M?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M a...
Questions in other subjects:

Mathematics, 11.07.2019 19:10









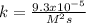
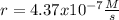
![r=k[NO]^2[O_2]](/tpl/images/0747/3983/b9fab.png)
![k=\frac{r}{[NO]^2[O_2]}\\\\k=\frac{0.00022022M/s}{(0.8M)^2(3.7M)} \\\\k=\frac{9.3x10^{-5}}{M^2s}](/tpl/images/0747/3983/c4bb6.png)