Chemistry, 09.09.2020 01:01 oscardiazbet8803
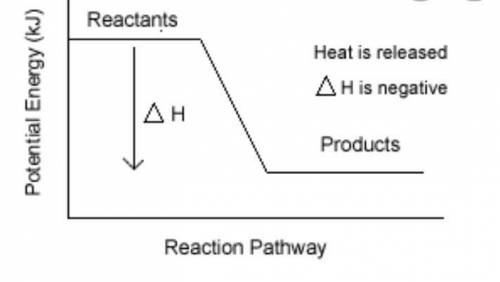
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the heat of reaction for the above reaction by using the following bond energies . (3 Marks) C-H single bond is 412 kJ , O=O double bond is 496 kJ, C=O double bond is 743 kJ, H-O single bond is 463 kJ b) Draw the energy profile diagram for the above reaction .On your diagram label the • Products, Reactants • enthalpy for the reaction • activation energy, Ea.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the hea...
Questions in other subjects:


Mathematics, 17.02.2020 21:03



History, 17.02.2020 21:03




Mathematics, 17.02.2020 21:04

Mathematics, 17.02.2020 21:04