
It is the balance between thermal energy and intermolecular forces that determines the state of matter of any substance at room temperature. How does thermal
energy increase as temperature is doubled?
1) Thermal energy increases by a factor of e (2.718)
2) Thermal energy increases by a factor of R
3)Thermal energy increases by a factor of ten
4)Thermal energy increases by a factor of two

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


You know the right answer?
It is the balance between thermal energy and intermolecular forces that determines the state of matt...
Questions in other subjects:

English, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Physics, 10.03.2021 14:00

History, 10.03.2021 14:00




Mathematics, 10.03.2021 14:00


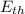 = Kinetic energy of translation + Kinetic energy of rotation + Energy of vibration
= Kinetic energy of translation + Kinetic energy of rotation + Energy of vibration


