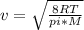Chemistry, 08.09.2020 15:01 trashbinkid
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure. For which gas do the molecules have the highest average velocity?
a) He
b) Cl2
c) all gases the same
d) NH3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure....
Questions in other subjects:

Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

Chemistry, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00


Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

History, 20.01.2021 07:00

History, 20.01.2021 07:00