
Chemistry, 08.09.2020 14:01 awesomegrill
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201 decays to a mercury isotope. Express your answer as a nuclear equation. Part CGold-198 undergoes beta emission. Express your answer as a nuclear equation. Part DGold-188 decays by positron emission. Express your answer as a nuclear equation.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

You know the right answer?
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201...
Questions in other subjects:

Mathematics, 31.03.2020 19:33

Mathematics, 31.03.2020 19:33



Chemistry, 31.03.2020 19:33



Mathematics, 31.03.2020 19:33


Mathematics, 31.03.2020 19:33

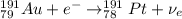
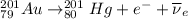
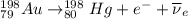
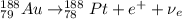
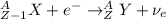
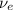 ) is emitted.
) is emitted. 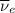 ) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant). 

