

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 23.06.2019 12:00, angieplasencia8
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
An electron in the n = 3 energy level of the hydrogen atom emits a photon with wavelength 656.27 nm....
Questions in other subjects:

Biology, 21.10.2019 20:30

Biology, 21.10.2019 20:30




Biology, 21.10.2019 20:30

Chemistry, 21.10.2019 20:30



Mathematics, 21.10.2019 20:30

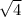 = 2
= 2

