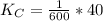Chemistry, 06.09.2020 02:01 Deavionaaaaa
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H2O(g) Kc=40NiO(s) +CO(g) ⇌ Ni(s) +CO2(g) Kc=600Calculate the value for the equilibrium constant, Kc, for the reaction:CO2(g) + H2(g) ⇌ CO(g) + H2O(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H...
Questions in other subjects:


Mathematics, 22.06.2021 02:00



Mathematics, 22.06.2021 02:00


English, 22.06.2021 02:00

Mathematics, 22.06.2021 02:00

Biology, 22.06.2021 02:00