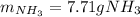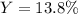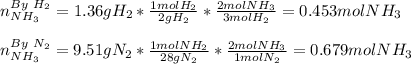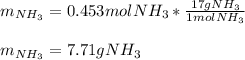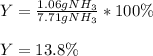Chemistry, 05.09.2020 22:01 Lindsay882
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is the theoretical yield in grams for this reaction under the given conditions? 2.)What is the percent yield for this reaction under the given conditions?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is...
Questions in other subjects:






History, 02.08.2019 16:00


Social Studies, 02.08.2019 16:00


Computers and Technology, 02.08.2019 16:00