Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 20jessicacabriales
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

You know the right answer?
At a certain temperature, 0.338 mol CH and 0.831 mol HO is placed in a 3.50 L container.
CH(g)+2HO(...
Questions in other subjects:

Biology, 15.04.2020 21:10


Mathematics, 15.04.2020 21:10

Mathematics, 15.04.2020 21:10

Geography, 15.04.2020 21:10


Mathematics, 15.04.2020 21:10



Biology, 15.04.2020 21:10

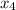 and 0.831 mol H
and 0.831 mol H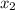 O is placed in a 3.50 L container.
CH
O is placed in a 3.50 L container.
CH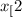 (g) At equilibrium, 6.51 g CO
(g) At equilibrium, 6.51 g CO