
Chemistry, 03.09.2020 07:01 qawsedrftgyh3487
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x 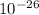 g, the total mass of its protons was 4.015 x
g, the total mass of its protons was 4.015 x 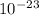 g, and the total mass of its neutrons was 4.690 x
g, and the total mass of its neutrons was 4.690 x 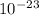 g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x
g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x 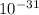 Proton one mass = 1.673 x
Proton one mass = 1.673 x 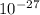 Neutron one mass = 1.675 x
Neutron one mass = 1.675 x 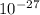

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3


Chemistry, 23.06.2019 11:30, nickolasbradyp0hvwl
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x...
Questions in other subjects:

Mathematics, 27.08.2019 14:30

Mathematics, 27.08.2019 14:30

Social Studies, 27.08.2019 14:30

Physics, 27.08.2019 14:30

Mathematics, 27.08.2019 14:30

Social Studies, 27.08.2019 14:30

Mathematics, 27.08.2019 14:30

Social Studies, 27.08.2019 14:30


Mathematics, 27.08.2019 14:30



