Chemistry, 02.09.2020 23:01 justhereforanswers13
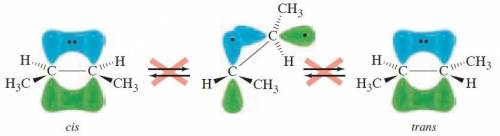
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p orbitals of the carbon-carbon π bond would be lost2) the double bond is much shorter and therefore more difficult to rotate3) the overlap of the sp2 orbitals of the carbon-carbon σ bond would be lost4) the double bond is much stronger and therefore more difficult to rotate

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p...
Questions in other subjects:


Social Studies, 31.07.2021 14:30


Health, 31.07.2021 14:30

Physics, 31.07.2021 14:30

Mathematics, 31.07.2021 14:30

Mathematics, 31.07.2021 14:30

Mathematics, 31.07.2021 14:30

Mathematics, 31.07.2021 14:30