Chemistry, 02.09.2020 20:01 olgapagan3173
What is the mass of a sample of iron that has had 300.0 j applied to it and heats up from 20.0 degrees Celsius to 40.0 degrees Celsius.. the specific heat of iron is 0.46j/gC

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
What is the mass of a sample of iron that has had 300.0 j applied to it and heats up from 20.0 degre...
Questions in other subjects:

Mathematics, 02.07.2019 20:00

Mathematics, 02.07.2019 20:00


Mathematics, 02.07.2019 20:00

Mathematics, 02.07.2019 20:00


Mathematics, 02.07.2019 20:00


Mathematics, 02.07.2019 20:00

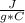 m= ?Tfinal= 40 CTinitial= 20 C
m= ?Tfinal= 40 CTinitial= 20 C