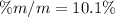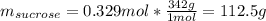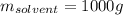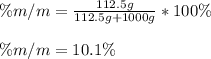Chemistry, 02.09.2020 04:01 Spoilmom1901
What is the mass percent of sucrose (C12H22O11, Mm = 342 g/mol) in a 0.329-m sucrose solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1
You know the right answer?
What is the mass percent of sucrose (C12H22O11, Mm = 342 g/mol) in a 0.329-m sucrose solution?...
Questions in other subjects:









Mathematics, 05.03.2020 23:37