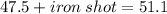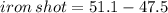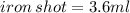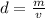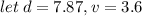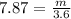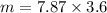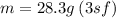Chemistry, 31.08.2020 07:01 milkshakegrande101
A sample of iron shot is added to a graduated cylinder containing 47.5 mL of water. The water level rises to the 51.1 mL mark. The density of iron is 7.87 g/mL. From this information, calculate the mass of the sample. *

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2



Chemistry, 23.06.2019 15:20, KhloodAhmed
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2
You know the right answer?
A sample of iron shot is added to a graduated cylinder containing 47.5 mL of water. The water level...
Questions in other subjects:


History, 06.07.2019 13:30

Health, 06.07.2019 13:30

History, 06.07.2019 13:30

Mathematics, 06.07.2019 13:30

Chemistry, 06.07.2019 13:30