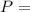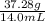Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3

You know the right answer?
A shiny chunk of metal is found to have a mass of 37.28g. The metal is dropped into a graduated cyli...
Questions in other subjects:

History, 04.05.2021 18:50

Mathematics, 04.05.2021 18:50



Health, 04.05.2021 18:50

Mathematics, 04.05.2021 18:50





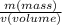 . Moreover, in this case, it is known the mass is 37.28 g, but the volume is not directly provided. However, we know the water in the graduated cylinder had a volume of 20.0 mL and this increased to 34.0 mL when the chunk of metal is added, this means the volume of the metal is 14 mL (34.0 mL - 20.0 mL = 14 mL). Now let's calculate the density:
. Moreover, in this case, it is known the mass is 37.28 g, but the volume is not directly provided. However, we know the water in the graduated cylinder had a volume of 20.0 mL and this increased to 34.0 mL when the chunk of metal is added, this means the volume of the metal is 14 mL (34.0 mL - 20.0 mL = 14 mL). Now let's calculate the density: