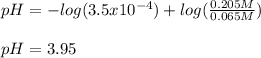Chemistry, 30.08.2020 14:01 jesussanchez1445
What is the pH of a buffer made from 0.130 mol of HCNO (Ka = 3.5 × 10⁻⁴) and 0.410 mol of NaCNO in 2.0 L of solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Thomas7785
Two things that biomedical has invented or innovated
Answers: 1


Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
What is the pH of a buffer made from 0.130 mol of HCNO (Ka = 3.5 × 10⁻⁴) and 0.410 mol of NaCNO in 2...
Questions in other subjects:

Mathematics, 22.12.2020 01:00


Arts, 22.12.2020 01:00

History, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00


Mathematics, 22.12.2020 01:00

History, 22.12.2020 01:00

Geography, 22.12.2020 01:00

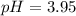
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0734/8563/33848.png)
![[base]=\frac{0.410mol}{2.0L}=0.205M](/tpl/images/0734/8563/eabfc.png)
![[acid]=\frac{0.130mol}{2.0L}=0.065M](/tpl/images/0734/8563/e375b.png)