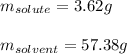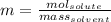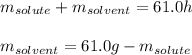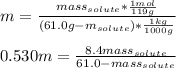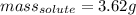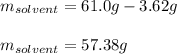Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
A 0.530 m aqueous solution of KBr has a total mass of 61.0 g. What masses of solute and solvent are...
Questions in other subjects:


Mathematics, 08.05.2021 15:30

English, 08.05.2021 15:30



Mathematics, 08.05.2021 15:30

Mathematics, 08.05.2021 15:30


English, 08.05.2021 15:30