
Chemistry, 30.08.2020 01:01 arturo698839
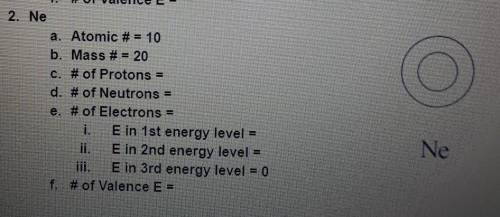
a. Atomic #= 3 b. Mass #=7 c. # of Protons = d. # of Neutrons = e. # of Electrons = E in Ist energy level = E in 2nd energy level = E in 3rd energy level = 0 # of Valence E = Li

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
a. Atomic #= 3 b. Mass #=7 c. # of Protons = d. # of Neutrons = e. # of Electrons = E in Ist energy...
Questions in other subjects:













