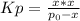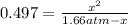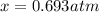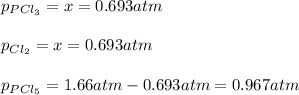Chemistry, 29.08.2020 23:01 mandilynn22
For the equilibrium PCl5(g)⇌PCl3(g)+Cl2(g), the equilibrium constant Kp is 0.497 at 500K. A gas cylinder at 500K is charged with PCl5(g) at an initial pressure of 1.66atm. Determine the equilibrium pressure of all species at this temperature.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
For the equilibrium PCl5(g)⇌PCl3(g)+Cl2(g), the equilibrium constant Kp is 0.497 at 500K. A gas cyli...
Questions in other subjects:


History, 04.05.2021 20:30

Mathematics, 04.05.2021 20:30

Mathematics, 04.05.2021 20:30


English, 04.05.2021 20:30



Social Studies, 04.05.2021 20:30

Arts, 04.05.2021 20:30

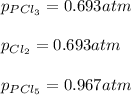
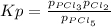
 can also be written as:
can also be written as: