B.) Positively charged

Chemistry, 28.08.2020 07:01 gbrbogdan9665
What is the bond character of this molecule?
A.) strongly covalent
B.) Positively charged
C.) Strongly ionic
D.) Negatively charged
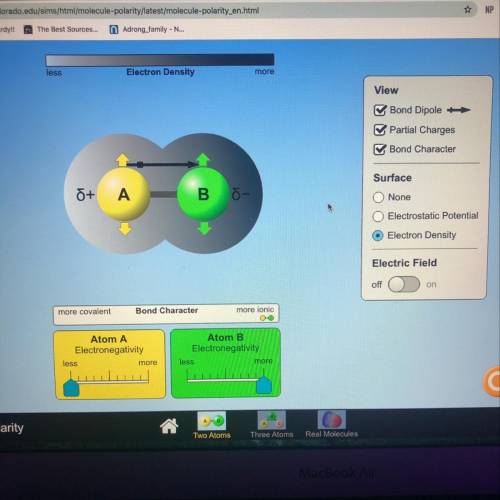

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
What is the bond character of this molecule?
A.) strongly covalent
B.) Positively charged
B.) Positively charged
Questions in other subjects:





Mathematics, 05.12.2020 04:40




Social Studies, 05.12.2020 04:40

History, 05.12.2020 04:40




