
Chemistry, 29.08.2020 07:01 Jackiebear4593
Which of the following represents an electron configuration that corresponds to the valence electrons of an element for which there is an especially large jump between the second and third ionization energies?
A. 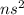
B. 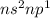
C. 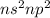
D. 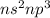

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Which of the following represents an electron configuration that corresponds to the valence electron...
Questions in other subjects:

Mathematics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50

Arts, 11.03.2021 02:50

Physics, 11.03.2021 02:50


History, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50





