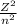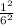Chemistry, 28.08.2020 23:01 naomicervero
From the Bohr equation in the introduction, the calculated energy of an electron in the sixth Bohr orbit of a hydrogen atom is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3


Chemistry, 23.06.2019 09:30, raymondmancilla123
The mass of a proton is approximately equal to the mass of
Answers: 1

Chemistry, 23.06.2019 09:40, 23rwilliamson
Is cutting your nails a physical or chemical change
Answers: 2
You know the right answer?
From the Bohr equation in the introduction, the calculated energy of an electron in the sixth Bohr o...
Questions in other subjects:


Social Studies, 27.06.2019 10:40


Mathematics, 27.06.2019 10:40


History, 27.06.2019 10:40