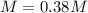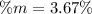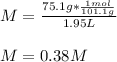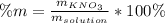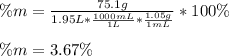Chemistry, 28.08.2020 23:01 journeyhile5
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L .Calculate the molarity of the solution. (assume a density of 1.05 g/mL for the solution)
Calculate the molality of the solution.
Calculate the mass percent of the solution.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 11:30, nickolasbradyp0hvwl
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L ....
Questions in other subjects:

History, 28.09.2019 23:30

Biology, 28.09.2019 23:30

Mathematics, 28.09.2019 23:30


Mathematics, 28.09.2019 23:30

Social Studies, 28.09.2019 23:30



English, 28.09.2019 23:30