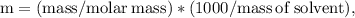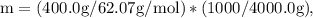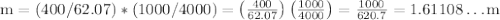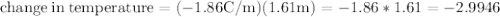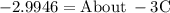Chemistry, 26.08.2020 23:01 keigleyhannah30
Ethylene glycol (C2H6O2) is used as an antifreeze in cars. If 400 g of ethylene glycol is added to 4.00 kg of water, what is the molality? Calculate how much the freezing point of water will be lowered. The freezing-point depression constant for water is Kf = –1.86°C/m.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2



Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
You know the right answer?
Ethylene glycol (C2H6O2) is used as an antifreeze in cars. If 400 g of ethylene glycol is added to 4...
Questions in other subjects:


Mathematics, 03.12.2019 15:31

Social Studies, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31

History, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31


Biology, 03.12.2019 15:31

Advanced Placement (AP), 03.12.2019 15:31

Mathematics, 03.12.2019 15:31