
Chemistry, 26.08.2020 18:01 blueval3tine
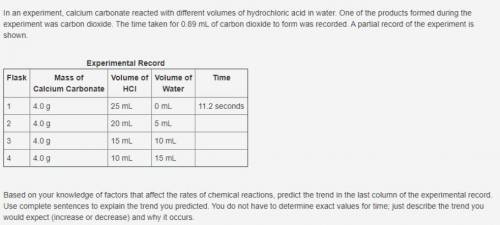
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in water. One of the products formed during the experiment was carbon dioxide. The time taken for 0.89 mL of carbon dioxide to form was recorded. A partial record of the experiment is shown. Experimental Record Flask Mass of Calcium Carbonate Volume of HCl Volume of Water Time 1 4.0 g 25 mL 0 mL 11.2 seconds 2 4.0 g 20 mL 5 mL 3 4.0 g 15 mL 10 mL 4 4.0 g 10 mL 15 mL

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in water. On...
Questions in other subjects:


English, 06.11.2019 13:31

Chemistry, 06.11.2019 13:31

Social Studies, 06.11.2019 13:31





Mathematics, 06.11.2019 13:31



