Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Please show some work For the reaction: NO(g) + 1/2 O2(g) → NO2(g) ΔH°rxn is -114.14 kJ/mol. Calcula...
Questions in other subjects:

Mathematics, 27.12.2020 09:40


Mathematics, 27.12.2020 09:40


Mathematics, 27.12.2020 09:40

Mathematics, 27.12.2020 09:40

Social Studies, 27.12.2020 09:40

History, 27.12.2020 09:50

Mathematics, 27.12.2020 09:50

Social Studies, 27.12.2020 09:50

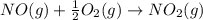
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0730/9939/db29b.png)
![\Delta H_{rxn}=[(n_{NO_2}\times \Delta H_f_{NO_2})]-[(n_{O_2}\times \Delta H_f_{O_2})+(n_{NO}\times \Delta H_f_{NO})]](/tpl/images/0730/9939/49ca0.png)
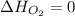 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-114.14=[(1\times 33.90)]-[(\frac{1}{2}\times 0)+(1\times \Delta H_f{NO})]](/tpl/images/0730/9939/4bbec.png)