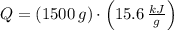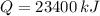Chemistry, 25.08.2020 02:01 Spencerg325
A certain chemical reaction releases 15.6/kJg of heat for each gram of reactant consumed. How can you calculate the heat produced by the consumption of 1.5kg of reactant? the math up. But don't do any of it. Just leave your answer as a math expression.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1



Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
A certain chemical reaction releases 15.6/kJg of heat for each gram of reactant consumed. How can yo...
Questions in other subjects:

English, 20.04.2020 19:22

Mathematics, 20.04.2020 19:22

Mathematics, 20.04.2020 19:22



History, 20.04.2020 19:22




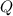 ), measured in kilojoules, is determined by the following expression:
), measured in kilojoules, is determined by the following expression: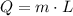
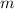 - Mass of reactant, measured in kilograms.
- Mass of reactant, measured in kilograms.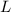 - Released heat ratio, measured in kilojoules per gram.
- Released heat ratio, measured in kilojoules per gram.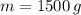 and
and 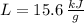 , the total energy released by chemical reaction is:
, the total energy released by chemical reaction is: