
Chemistry, 21.08.2020 20:01 haleyrene3924
Consider two bulbs seperated by a valce. Both bulbs are amintained at the same temperature. Assume that when the valve between the two bulbs is closed, the gases are sealed in their respective bulbs. When the valve is closed, the following data apply:
Bulb A Bulb B
Gas Ne CO
V 2.50L 2.00L
P 1.09 atm 0.73 atm
Assuming no temperature change, determine the final pressure inside the system after the valve connecting the two bulbs is opened. Ignore the volume of the tube connecting the two bulbs.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

You know the right answer?
Consider two bulbs seperated by a valce. Both bulbs are amintained at the same temperature. Assume t...
Questions in other subjects:

History, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01

Social Studies, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

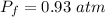
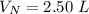
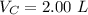
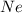 is
is 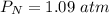
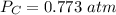
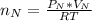
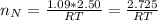
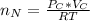
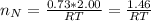
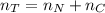
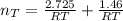
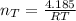
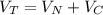
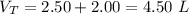
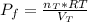
![P_f = \frac{[\frac{4.185}{RT} ] * RT }{ 4.50}](/tpl/images/0726/7070/5fdbe.png)



