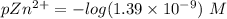Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
Calculate the pZn of a solution prepared by mixing 25.0 mL of 0.0100 M EDTA with 50.0 mL of 0.00500...
Questions in other subjects:

Health, 18.11.2020 18:30

Mathematics, 18.11.2020 18:30

English, 18.11.2020 18:30

English, 18.11.2020 18:30




SAT, 18.11.2020 18:30

Mathematics, 18.11.2020 18:30

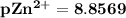
![pH = pKa[NH^+_4] + log \dfrac{[NH_3]}{[NH_4^+]}](/tpl/images/0726/1828/94d35.png)
 = 9.26
= 9.26 = 0.100 M
= 0.100 M 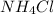 = 0.176 M
= 0.176 M![pH = 9.26 + log \dfrac{[0.100]}{[0.176]}](/tpl/images/0726/1828/11e21.png)
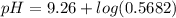
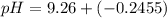
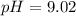
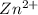 and EDTA is :
and EDTA is :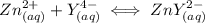
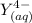 denotes the fully deprotonated form of the EDTA
denotes the fully deprotonated form of the EDTA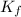 of the equation for the reaction can be represented as:
of the equation for the reaction can be represented as:![K_f = \dfrac{[ZnY^{2-}]}{[Zn^{2+} ][Y^{4-}]}](/tpl/images/0726/1828/c7b1d.png) ----- (1)
----- (1)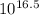
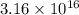
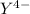 ,
,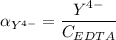
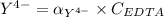
![K_f = \dfrac{[ZnY^{2-}]}{[Zn^{2+} ] \ \ \alpha_ {Y^{4-}} \times {C_{EDTA}}}](/tpl/images/0726/1828/75df9.png)
![K_f' = K_f \times \alpha _Y{^4-} = \dfrac{[ZnY^{2-}]}{[Zn^{2+} ] \ C_{EDTA} }](/tpl/images/0726/1828/a3102.png)
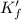 = conditional formation constant
= conditional formation constant 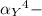 = the fraction of EDTA that exit in the form of the presences of the 4 charges .
= the fraction of EDTA that exit in the form of the presences of the 4 charges .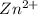 initially in titrand is now present in
initially in titrand is now present in 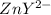
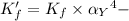
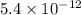
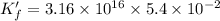
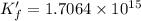
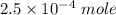
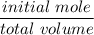
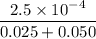
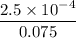
![K_f' = \dfrac{[ZnY^{2-}]}{[Zn^{2+} ] \ C_{EDTA} }](/tpl/images/0726/1828/c89c2.png)
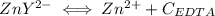
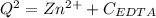
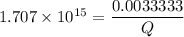
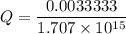
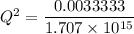
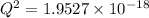
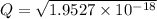
 M
M![[Zn^{2+}]= 1.39 \times 10^{-9} \ M](/tpl/images/0726/1828/63850.png)
![pZn ^{2+} =- log [Zn^{2+}]](/tpl/images/0726/1828/3ecce.png)