
Chemistry, 20.08.2020 22:01 brendaslater49p6ttxt
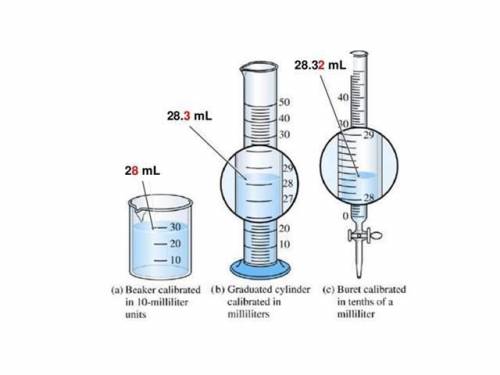
Bill, a lab technician, was asked to measure out sodium hydroxide (NaOH) for an experiment in a research lab. He was given a choice between a beaker, a graduated cylinder, and a buret (shown left). Bill used a graduated cylinder to measure the liquid NaOH because he says, “a graduated cylinder is the most accurate way to measure a liquid!” Do you agree or disagree with Bill? Use significant figures to justify your answer.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Bill, a lab technician, was asked to measure out sodium hydroxide (NaOH) for an experiment in a rese...
Questions in other subjects:

Social Studies, 31.03.2021 03:40

Biology, 31.03.2021 03:40



English, 31.03.2021 03:40

Business, 31.03.2021 03:40

Mathematics, 31.03.2021 03:40

Mathematics, 31.03.2021 03:40

Mathematics, 31.03.2021 03:40

Biology, 31.03.2021 03:40



