
Chemistry, 18.08.2020 17:01 cluchmasters5634
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reaction mixture have on the system?
CuS(s) + O2(g) ? Cu(s) + SO2(g)
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reaction mixture have on the system?
CuS(s) + O2(g) ? Cu(s) + SO2(g)
a) The equilibrium constant will increase.
b) The reaction will shift to the left in the direction of reactants.
c) The reaction will shift to the right in the direction of products.
d) No effect will be observed.
e) The equilibrium constant will decrease.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

You know the right answer?
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reac...
Questions in other subjects:

Biology, 04.08.2019 15:00

Social Studies, 04.08.2019 15:00

History, 04.08.2019 15:00


Mathematics, 04.08.2019 15:00



Mathematics, 04.08.2019 15:00


Mathematics, 04.08.2019 15:00

![Q = \displaystyle \frac{[\mathrm{SO_2\, (g)}]}{[\mathrm{O_2\, (g)}]}](/tpl/images/0723/8610/a7d3d.png) .
.![[\mathrm{SO_2\, (g)}]](/tpl/images/0723/8610/2691f.png) and
and ![[\mathrm{O_2\, (g)}]](/tpl/images/0723/8610/46794.png) will increase if the pressure is increased through compression. However, because
will increase if the pressure is increased through compression. However, because 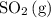 and
and  have the same coefficients in the equation, their concentrations are raised to the same power in the equilibrium quotient
have the same coefficients in the equation, their concentrations are raised to the same power in the equilibrium quotient 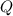 .
.  . If the system was already at equilibrium, it will continue to be at an equilibrium even after the change to its pressure. Therefore, no overall effect on the equilibrium position should be visible.
. If the system was already at equilibrium, it will continue to be at an equilibrium even after the change to its pressure. Therefore, no overall effect on the equilibrium position should be visible.

