
Chemistry, 19.08.2020 02:01 markuswalter1043
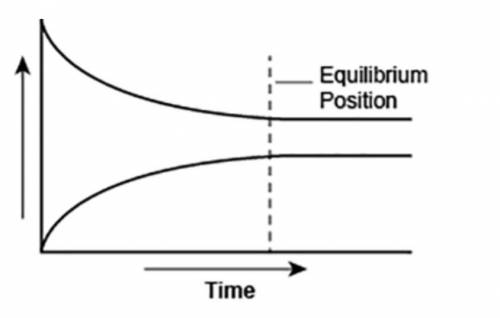
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot to label the y-axis of the graph.
What best explains the label that the student should use on the y-axis? A. Concentration, because as the amount of product decreases, the amount of reactant increases over time.
B. Reaction rate, as the rates of forward and backward reactions become equal at equilibrium.
C. Concentration, because the amounts of reactants and products remain constant after equilibrium is reached.
D. Reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot...
Questions in other subjects:


Spanish, 27.09.2019 23:00



Mathematics, 27.09.2019 23:00


Chemistry, 27.09.2019 23:00

Physics, 27.09.2019 23:00




