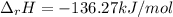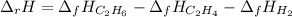Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Using standard heats of formation, calculate the standard enthalpy change for the following reaction...
Questions in other subjects:




English, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00


Social Studies, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

English, 23.02.2021 21:00

Arts, 23.02.2021 21:00