
Chemistry, 18.08.2020 20:01 epmooneyham922
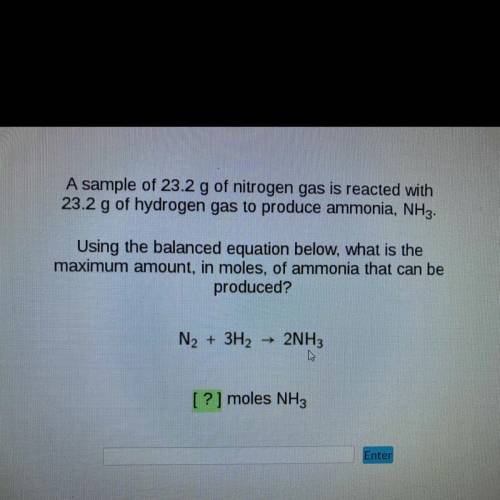
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.
Using the balanced equation below, what is the
maximum amount, in moles, of ammonia that can be
produced?
N2 + 3H2
2NH3
IN
[?] moles NH3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.<...
Questions in other subjects:



Mathematics, 31.10.2019 20:31

History, 31.10.2019 20:31



Physics, 31.10.2019 20:31





