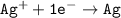Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
Write a chemical equation for the reaction that occurs in the following cell: Cu|Cu2+(aq)||Ag+(aq)|A...
Questions in other subjects:

Mathematics, 22.03.2021 05:40



Mathematics, 22.03.2021 05:40

Mathematics, 22.03.2021 05:40

Mathematics, 22.03.2021 05:40

Mathematics, 22.03.2021 05:40

Mathematics, 22.03.2021 05:40


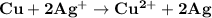
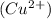 which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode
which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode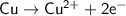
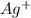 becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.
becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.