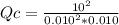Chemistry, 16.08.2020 01:01 longoriafaithe09
At 700 K, the reaction 2SO 2(g) + O 2(g) 2SO 3(g) has the equilibrium constant K c = 4.3 × 10 6, and the following concentrations are present: [SO 2] = 0.010 M; [SO 3] = 10. M; [O 2] = 0.010 M. Which of the following is true based on the above?
A. Qc < Kc, the reaction proceeds from right to left to reach equilibrium
B. Qc < Kc, the reaction proceeds from left to right to reach equilibrium
C. Qc > Kc, the reaction proceeds from right to left to reach equilibrium
D. Qc > Kc, the reaction proceeds from left to right to reach equilibrium
E. Qc = Kc, the reaction is currently at equilibriums

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3
You know the right answer?
At 700 K, the reaction 2SO 2(g) + O 2(g) 2SO 3(g) has the equilibrium constant K c = 4.3 × 10 6, and...
Questions in other subjects:

History, 18.10.2019 12:00

Mathematics, 18.10.2019 12:00

History, 18.10.2019 12:00

Social Studies, 18.10.2019 12:00





Computers and Technology, 18.10.2019 12:00

History, 18.10.2019 12:00

![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0723/0556/1eb27.png)
![Qc=\frac{[SO_{3} ]^{2} }{[SO_{2} ]^{2} *[O_{2} ]}](/tpl/images/0723/0556/691d7.png)