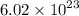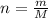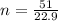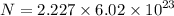Chemistry, 15.08.2020 21:01 Ellafrederick
Determine the number of atoms in 51.0 grams of sodium, Na. (The mass of one mole of sodium is 22.99 g.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
You know the right answer?
Determine the number of atoms in 51.0 grams of sodium, Na. (The mass of one mole of sodium is 22.99...
Questions in other subjects:


Social Studies, 29.10.2019 02:31


Biology, 29.10.2019 02:31

Mathematics, 29.10.2019 02:31

Mathematics, 29.10.2019 02:31

Mathematics, 29.10.2019 02:31

English, 29.10.2019 02:31


History, 29.10.2019 02:31