
Chemistry, 15.08.2020 08:01 kingofmortals2119
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at de-icing roads in the winter? A. Calcium chloride only breaks into 2 ions, while sodium chloride does not break up and remains 1 ion. B. Calcium is a larger molecule than sodium, so it has a greater effect. C. The additional chlorine atom forms a stronger bond between the atoms in calcium chloride than in sodium chloride, so calcium chloride remains one molecule while sodium chloride breaks apart. D. Calcium chloride breaks into 3 ions, while sodium chloride only breaks into 2 ions.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 03:30, jennelledenise
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at...
Questions in other subjects:



History, 14.04.2021 20:40

English, 14.04.2021 20:40

Mathematics, 14.04.2021 20:40



Mathematics, 14.04.2021 20:40

Mathematics, 14.04.2021 20:40

Mathematics, 14.04.2021 20:40

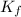 × m × i
× m × i

