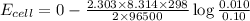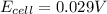Chemistry, 13.08.2020 18:01 scottcounts757
A concentration cell is one in which both the anode and cathode are the same but with different concentrations. Calculate the cell potential with [Zn2+] = 0.10 M[Zn2+] = 0.10 M for the cathode and the [Zn2+] = 0.010 M[Zn2+] = 0.010 M for the anode?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
A concentration cell is one in which both the anode and cathode are the same but with different conc...
Questions in other subjects:




Mathematics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30


English, 14.05.2021 22:30


Health, 14.05.2021 22:30

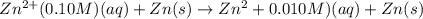
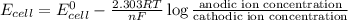
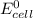 = standard electrode potential of the cell = 0 (as both metals are same )
= standard electrode potential of the cell = 0 (as both metals are same )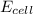 = emf of the cell = ?
= emf of the cell = ?