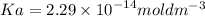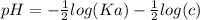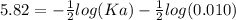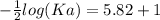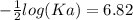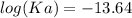Chemistry, 13.08.2020 17:01 lucifer6669
The pH of an acid solution is 5.82. Calculate the Ka for the monoprotic acid. The initial acid concentration is 0.010 M.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
The pH of an acid solution is 5.82. Calculate the Ka for the monoprotic acid. The initial acid conce...
Questions in other subjects:

Health, 27.08.2020 03:01

History, 27.08.2020 03:01

English, 27.08.2020 03:01

English, 27.08.2020 03:01