

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 16:30, aloading2256
Answer immediately when two or more atoms of the same element are in a chemical bond, the substance is called a(n) a. compound b. molecule c. nucleus
Answers: 1
You know the right answer?
At 25 °C, only 0.0990 mol of the generic salt AB3 is soluble in 1.00 L of water. What is the Ksp of...
Questions in other subjects:


Mathematics, 16.02.2022 07:40

Social Studies, 16.02.2022 07:40





SAT, 16.02.2022 07:40

SAT, 16.02.2022 07:40

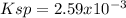

![[A]=0.099 \frac{molAB_3}{L}*\frac{1molA}{1molAB_3} =0.0099M](/tpl/images/0722/0213/dd0c8.png)
![[B]=0.099 \frac{molAB_3}{L}*\frac{3molB}{1molAB_3} =0.000.297M](/tpl/images/0722/0213/411cb.png)
![Ksp=[A][B]^3](/tpl/images/0722/0213/9740b.png)
![Ksp=[0.099M][0.297M]^3\\\\Ksp=2.59x10^{-3}](/tpl/images/0722/0213/a2275.png)


