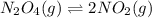Chemistry, 14.08.2020 04:01 sillslola816oxb5h7
If we represent the equilibrium as:...N2O4(g) 2 NO2(g) We can conclude that: 1. This reaction is: A. Exothermic B. Endothermic C. Neutral D. More information is needed to answer this question. 2. When the temperature is increased the equilibrium constant, K: A. Increases B. Decreases C. Remains the same D. More information is needed to answer this question. 3. When the temperature is increased the equilibrium concentration of NO2: A. Increases B. Decreases C. Remains the same D. More information is needed to answer this question.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 23.06.2019 09:30, alexabdercmur
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

Chemistry, 23.06.2019 12:50, jasonoliva13
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
If we represent the equilibrium as:...N2O4(g) 2 NO2(g) We can conclude that: 1. This reaction is: A....
Questions in other subjects:

Chemistry, 30.01.2020 00:58

Biology, 30.01.2020 00:58

English, 30.01.2020 00:58

English, 30.01.2020 00:58

Chemistry, 30.01.2020 00:58



Chemistry, 30.01.2020 00:58


Mathematics, 30.01.2020 00:58