

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1


Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1

Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
the ka of hypochlorous acid (hclo) is 3.0 x10^-8 at 25.0°C. What is the % of ionization of hypochlor...
Questions in other subjects:

Physics, 16.12.2020 08:00

Mathematics, 16.12.2020 08:00

Mathematics, 16.12.2020 08:00

Mathematics, 16.12.2020 08:00

Mathematics, 16.12.2020 08:00


Chemistry, 16.12.2020 08:00

Arts, 16.12.2020 08:00


![Ka = \frac{[H+][ClO-]}{[HClO]}](/tpl/images/0721/9838/ef45a.png)
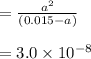
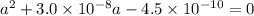
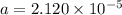
![[H+] = a = 2.120 \times 10^{-5} M](/tpl/images/0721/9838/b6a0d.png)
![= \frac{[H+]}{[HClO]}_{initial} \times 100\%\\\\= 2.120 \times 10^{-5}\div0.015 \times 100\%](/tpl/images/0721/9838/3381a.png)


