Chemistry, 14.08.2020 04:01 madpanda55
f a substance has a half-life of 8.10 hr, how many hours will it take for 75.0 g of the substance to be depleted to 3.90 g?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2



Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
f a substance has a half-life of 8.10 hr, how many hours will it take for 75.0 g of the substance to...
Questions in other subjects:


English, 04.11.2020 21:40



Health, 04.11.2020 21:40



Mathematics, 04.11.2020 21:40


Mathematics, 04.11.2020 21:40

 = 8.1 hr
= 8.1 hr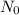 = 75 g
= 75 g = 3.9 g
= 3.9 g = ?
= ?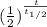
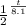
 log 1/2
log 1/2