
Chemistry, 12.08.2020 08:01 christabell0303
5. Two reactants combine to form a product in the reaction A + B -> C. The rate of the reaction depends on the concentrations of both
reactants squared (rate = k[A]2 [B]2). What's the total reaction order of this reaction?
A. 4
B. 1
C. 2.
O D.3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
5. Two reactants combine to form a product in the reaction A + B -> C. The rate of the reaction d...
Questions in other subjects:

Mathematics, 01.06.2021 19:50

Mathematics, 01.06.2021 19:50



Biology, 01.06.2021 19:50

Mathematics, 01.06.2021 19:50



Mathematics, 01.06.2021 19:50

Mathematics, 01.06.2021 19:50

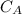 and
and  are the concentrations of A and B respectively at any time t, then assuming that the reaction is of first order with respect to both A and B , the overall order is second and the reaction rate is given by:
are the concentrations of A and B respectively at any time t, then assuming that the reaction is of first order with respect to both A and B , the overall order is second and the reaction rate is given by: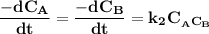
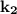 = specific rate constant for a second order reaction and becomes the rate of the reaction when both
= specific rate constant for a second order reaction and becomes the rate of the reaction when both 

