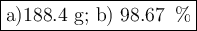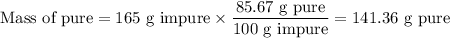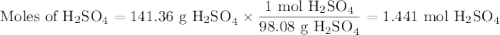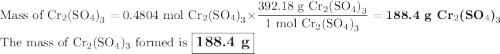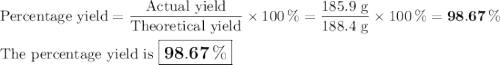Chromium is dissolved in sulfuric acid according to the following equation: Cr + H2SO4 ⇒ Cr2 (SO4) 3 + H2
a) How many grams of Cr2 (SO4) 3 can be obtained by reacting 165 g of 85.67% H2SO4 of purity?
b) If 485.9 g of Cr2 (SO4) 3 are obtained, what is the yield of the reaction?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Chromium is dissolved in sulfuric acid according to the following equation: Cr + H2SO4 ⇒ Cr2 (SO4) 3...
Questions in other subjects: