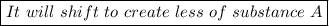PLEASE HELP WILL GIVE BRAINLIEST
If the concentration of substance A of a reversible reaction in dynamic equilibrium increases, how will the equilibrium change?
A. It will shift to create more of substance A.
B. It will shift towards the reactants.
C. It will shift towards the products.
D. It will shift to create less of substance A.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1



You know the right answer?
PLEASE HELP WILL GIVE BRAINLIEST
If the concentration of substance A of a reversible reaction in dy...
Questions in other subjects:

Mathematics, 25.02.2021 23:50

Chemistry, 25.02.2021 23:50



Physics, 25.02.2021 23:50



Mathematics, 25.02.2021 23:50


Biology, 25.02.2021 23:50