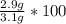Chemistry, 12.08.2020 07:01 kokokakahi
A student carries out the precipitation reaction shown below, starting with 0.030 moles of calcium nitrate. The final mass of the precipitate is 2.9 g. Answer the questions below to determine the percent yield. 3Ca(NO3)2(aq) + 2Na3PO4(aq) → Ca3(PO4)2(s) + 6NaNO3(aq) 1. a. Which product is the precipitate? b. How many moles of the precipitate would one expect to be produced from 0.030 moles of calcium nitrate? c. How many grams of solid do you expect to be produced? d. What is the percent yield?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
A student carries out the precipitation reaction shown below, starting with 0.030 moles of calcium n...
Questions in other subjects:

Chemistry, 08.07.2021 07:50


Mathematics, 08.07.2021 07:50


Mathematics, 08.07.2021 07:50


Mathematics, 08.07.2021 07:50

Mathematics, 08.07.2021 07:50