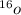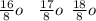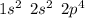. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with mass #’s 16, 17 and 18 i. What is the naturally occurring diatomic element ? (2 points) ii. Write the isotopic symbols. (2 points) iii. How many neutrons does each possess? (2 points) iv. What is the electronic configuration of each isotope?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with m...
Questions in other subjects:

History, 16.10.2020 08:01

History, 16.10.2020 08:01



Computers and Technology, 16.10.2020 08:01


Biology, 16.10.2020 08:01



Biology, 16.10.2020 08:01