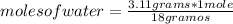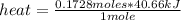Chemistry, 12.08.2020 04:01 zanaplen27
The heat of vaporization of water is 40.66 kJ/mol. How much heat is absorbed when 3.11 g of water boils at atmospheric pressure?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
The heat of vaporization of water is 40.66 kJ/mol. How much heat is absorbed when 3.11 g of water bo...
Questions in other subjects:





Social Studies, 19.10.2020 04:01

Mathematics, 19.10.2020 04:01

Biology, 19.10.2020 04:01